Peyton Fleagle was only a toddler when his mother and father first seen itchy scales seem on his pores and skin. Finally, the scales lined 95% of Peyton’s physique.
Fleagle, who’s now 12, has a situation referred to as X-linked ichthyosis, one in all an often-disfiguring group of pores and skin ailments that develop in kids.
Recognized collectively as congenital ichthyosis, the ailments are characterised by dry, scaly patches of pores and skin that cowl the physique. Ichthyosis impacts the best way the pores and skin regenerates, both by slowing down the shedding of previous pores and skin cells or rushing up the formation of recent pores and skin cells. There may be at present no therapy for ichthyosis accredited by the U.S. Meals and Drug Administration.
However that may change.
Yale is a testing web site for a possible new treatment—an ointment developed by Timber Prescribed drugs. The therapy works by affecting retinoic acid receptors within the nucleus of pores and skin cells. The therapy normalizes the turnover of pores and skin cells so that there’s much less retention of previous cells and fewer hyperactivity of pores and skin manufacturing.
For Peyton Fleagle, who’s a part of a part three medical trial for the brand new therapy at Yale New Haven Hospital, the indicators are encouraging. He skilled dramatic outcomes as a participant within the earlier medical trial for the ointment.
“It was like magic,” stated his mom Christina Fleagle, who lives in Agawam, Massachusetts. “His pores and skin fully cleared up. All of the scales had been gone.”
“After I’m not itching, I simply really feel completely different. I really feel higher, as a result of I am not consistently scratching,” Peyton stated.
Christopher Bunick, affiliate professor of dermatology at Yale College of Drugs and principal investigator for medical trials of the brand new therapy at Yale, stated Peyton’s expertise just isn’t distinctive.
“A number of of my sufferers have seen transformative outcomes, the place their scale is completely clear, the pores and skin clear for the primary time. It’s outstanding,” Bunick stated. “The research present that when sufferers use the medication as indicated, most of them present enchancment. It’s not a remedy, nonetheless.”
Bunick is corresponding creator of a trio of research, together with one that can quickly be revealed, reporting the outcomes of the medical trials. Bunick additionally introduced the trial outcomes at a gathering of the American Academy of Dermatology final yr.
The research checked out part two medical trials (there are three phases of trials earlier than potential FDA approval) centered on the security, tolerability, and efficacy of the brand new medication for treating sufferers.
The primary examine, within the Journal of the American Academy of Dermatology (JAAD), featured 19 sufferers and examined two focus ranges of the therapy for security and tolerability. Each concentrations had been discovered to be protected, however the decrease focus proved to be extra efficacious.
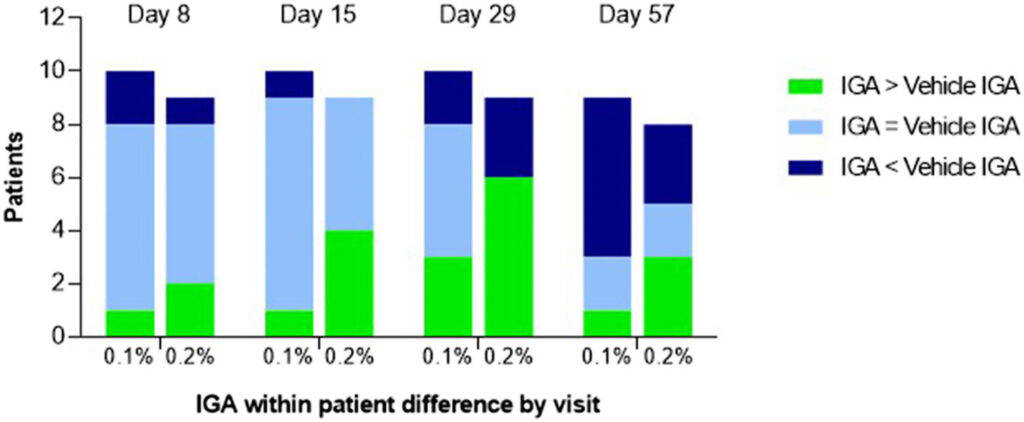
The second examine, additionally revealed in JAAD, included 33 sufferers and centered on the effectiveness of the therapy utilizing two fundamental measures: the Visible Index for Ichthyosis Severity (VIIS) scale system, which was developed by Keith Choate, chair of Yale’s Division of Dermatology, and the Investigator World Evaluation (IGA) rating, a five-point scale of illness standing for a spread of ailments. All sufferers who obtained a 0.05% focus of the therapy noticed a 50% discount in VIIS and two or extra ranges of enchancment in IGA.
The newest examine, which has been accepted by the journal Scientific and Experimental Dermatology, centered on the effectiveness of the brand new medication for treating two particular sorts of congenital ichthyosis: lamellar ichthyosis and X-linked recessive ichthyosis. The researchers discovered that the therapy labored equally effectively in each illness varieties, with all sufferers assembly the VIIS and IGA objectives.
Yale’s involvement within the medical trials—it’s the solely U.S. web site concerned in all three trials—is simply the newest instance of the college’s prominence in ichthyosis analysis. Emeritus professor of dermatology Leonard Milstone launched an ichthyosis clinic at Yale greater than 20 years in the past. In 2017, Keith Choate based an ichthyosis registry at Yale and—with Milstone and different colleagues—developed the VIIS scale system.
“The deep roots of ichthyosis analysis and medical care at Yale, and now through the medical trials, are on the verge of serving to convey to ichthyosis sufferers the primary FDA-approved remedy,” Bunick stated.
Lots of these sufferers, Bunick stated, expertise emotional and psychosocial trauma along with the illness’s bodily results.
“From a younger age sufferers should endure persistent fissured and scaly pores and skin, typically purple and infected as effectively, making them targets for jokes or bullying at college,” Bunick stated. “These sufferers are at all times ‘completely different’ on account of their look. They’ve fixed shedding of scales of their bedding, their clothes—mother and father endure that, too.”
For the Fleagle household, ichthyosis has meant a decade of fear, frustration, Epsom salt baths, lotions, and oils. Christina Fleagle stated different relations, together with her father and uncle, even have X-linked ichthyosis, however son Peyton’s case is by far essentially the most extreme.
“His pores and skin will get so dry, particularly within the winter when the heater is operating, that it looks like tissue paper and has these purple traces,” Christina Fleagle stated. “Peyton used to name it ‘cracking.'”
Bunick stated ichthyosis additionally makes wound therapeutic extra sophisticated for sufferers, who’re susceptible to an infection and gradual therapeutic.
The subsequent step in Bunick’s analysis will concentrate on the part three medical trial at Yale, which is actively enrolling members. Bunick additionally lauded the contributions of Mahin Dawood-Saffa and Nicole Olszewski for his or her work with the Yale Middle for Scientific Investigation to handle varied phases of the trials.
Extra data:
Amy S. Paller et al, Security, tolerability, and efficacy of a novel topical isotretinoin formulation for the therapy of X-linked or lamellar congenital ichthyosis: Outcomes from a part 2a proof-of-concept examine, Journal of the American Academy of Dermatology (2022). DOI: 10.1016/j.jaad.2022.02.060
Joyce M.C. Teng et al, The CONTROL examine: A randomized, double-blind vehicle-controlled part 2b examine of novel topical isotretinoin formulation demonstrates enchancment in recessive X-linked and autosomal recessive lamellar congenital ichthyosis, Journal of the American Academy of Dermatology (2022). DOI: 10.1016/j.jaad.2022.07.028
Yale College
Quotation:
Dermatologists check a promising therapy for pores and skin ailments that usually goal kids (2023, February 10)
retrieved 10 February 2023
from https://medicalxpress.com/information/2023-02-dermatologists-treatment-skin-diseases-children.html
This doc is topic to copyright. Other than any honest dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for data functions solely.