It solely takes holding your breath for barely too lengthy to know that too little oxygen is unhealthy for you. However are you able to even have an excessive amount of? Certainly, respiration air with the next oxygen stage than your physique wants could cause well being issues and even loss of life.
However with scant analysis on the subject, scientists have recognized little about how the physique senses an excessive amount of oxygen. Now, a brand new research from Gladstone Institutes has tremendously expanded the scientific physique of data in regards to the mechanisms at play, and why it issues for well being.
Their findings, reported within the journal Science Advances, clarify how respiration air with completely different ranges of oxygen—from too little, to only proper, or an excessive amount of—impacts the creation and degradation of various proteins within the lungs, coronary heart, and mind of mice. Notably, the research additionally highlights a specific protein that will play a central function in regulating how cells reply to hyperoxia.
“These outcomes have implications for a lot of completely different ailments,” says Gladstone Assistant Investigator Isha Jain, Ph.D., senior creator of the brand new research. “Greater than 1 million folks within the US breathe supplemental oxygen day-after-day for medical causes, and research counsel it may very well be making issues worse in some circumstances. That is only one setting the place our work is beginning to clarify what’s taking place and the way the physique responds.”
Understanding oxygen’s results
Most prior analysis on oxygen ranges has examined the molecular results of too little oxygen. And even in that realm, a lot of the focus has been on how low oxygen impacts which genes are turned on or off.
“Our research enters uncharted territory through the use of mice and looking out downstream of gene expression at which proteins abnormally accumulate or degrade in response to completely different oxygen concentrations,” says Kirsten Xuewen Chen, first creator of the brand new paper and a graduate pupil at UC San Francisco.
The analysis builds on the group’s prior work, which revealed that in response to an excessive amount of oxygen, sure proteins containing iron and sulfur clusters change into degraded, main cells to malfunction.
“Now, we wished to get a extra dynamic image of how proteins are regulated when oxygen ranges are too excessive or too low,” Chen says.
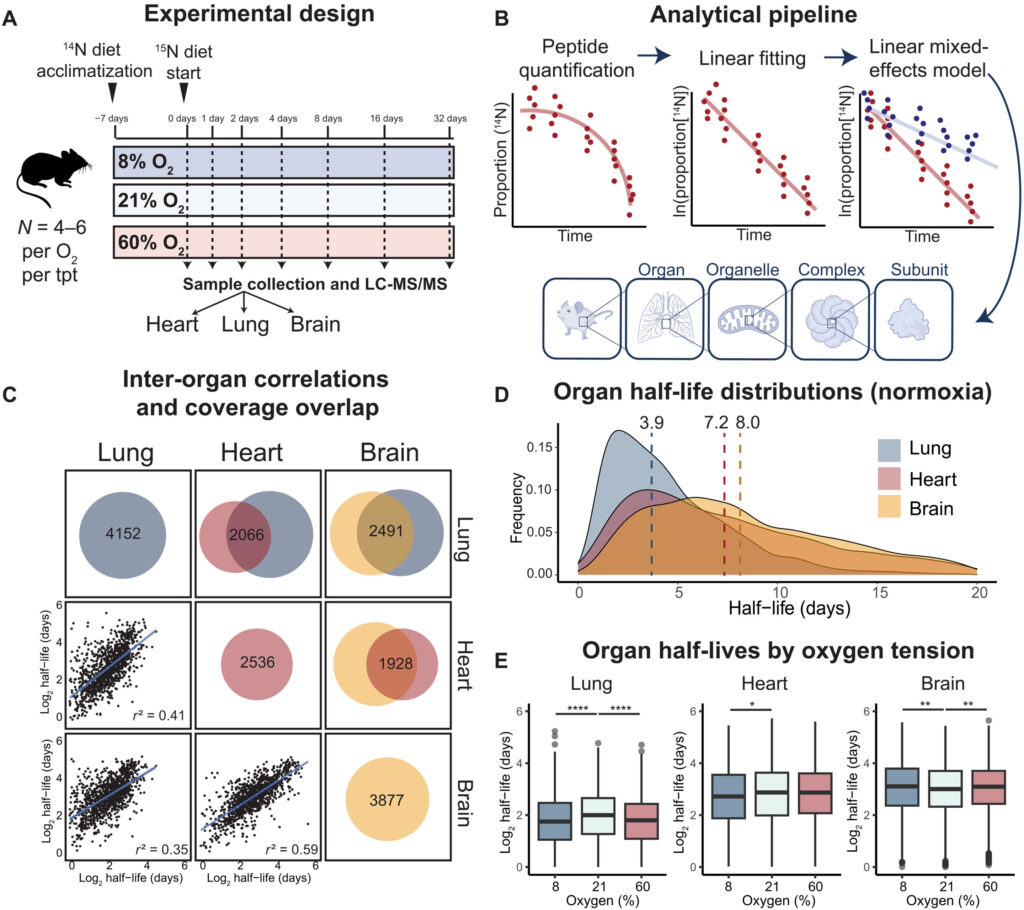
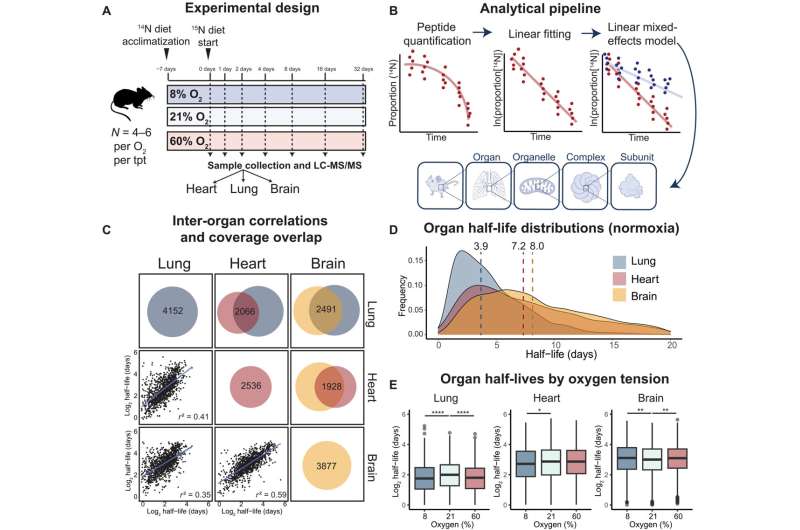
To take action, the group uncovered mice for a number of weeks to air with oxygen stage of 8 p.c, 21 p.c (the same old stage we breathe in Earth’s ambiance), or 60 p.c. In the meantime, they gave the mice meals containing a definite type of nitrogen that the animals’ our bodies integrated into new proteins. This nitrogen isotope acted as a “label” that enabled the researchers to calculate protein turnover charges—the steadiness between protein synthesis and degradation—for 1000’s of various proteins within the lungs, coronary heart, and mind.
“We’re grateful to our collaborators who’re the specialists on this approach, often known as secure isotope labeling of amino acids in mice,” Jain says. “With out it, we couldn’t have performed this research.”
A key protein builds up
The researchers discovered that oxygen ranges extra dramatically affected proteins within the lungs of mice than the center or mind. They recognized sure proteins with irregular turnover charges phys.org/information/2023-03-oxygen-c … ls-tissues.htmlunder high- or low-oxygen circumstances.
One specific protein that collected in high-oxygen circumstances, MYBBP1A, piqued their consideration. MYBBP1A is a transcription regulator, which means it instantly impacts gene expression.
“This caught our eye as a result of prior analysis has proven that different transcription elements referred to as hypoxia-inducible elements, or HIFs, play a giant function in cells’ response to low oxygen,” Chen says. “Our work nominates MYBBP1A for a associated function in hyperoxia signaling.”
MYBBP1A is concerned within the manufacturing of ribosomes—mobile “machines” that construct proteins. Additional experiments surfaced clues that, in response to excessive oxygen ranges, accumulation of this protein within the lungs might have an effect on manufacturing of ribosomal RNA, a key part of ribosomes.
Jain’s group is now analyzing the exact molecular function of MYBBP1A throughout hyperoxia, together with whether or not its response is protecting or dangerous. This work may set the stage for novel therapies that concentrate on the MYBBP1A protein or related molecules in ways in which counter the unhealthy results of hyperoxia—much like widespread analysis efforts focusing on HIF proteins in low-oxygen circumstances.
The brand new research presents a first-of-its-kind dataset of protein turnover charges in several tissues of mice uncovered to completely different oxygen ranges. The group hopes its outcomes will encourage different researchers to additional examine the consequences of an excessive amount of or too little oxygen on the physique, which may remodel the way in which we deal with illness.
Extra info:
Xuewen Chen et al, In Vivo Protein Turnover Charges in Various Oxygen Tensions Nominate MYBBP1A as a Mediator of the Hyperoxia Response, Science Advances (2023). DOI: 10.1126/sciadv.adj4884. www.science.org/doi/10.1126/sciadv.adj4884
Gladstone Institutes
Quotation:
New analysis paints a dynamic image of how we reply to excessive or low oxygen ranges (2023, December 8)
retrieved 14 December 2023
from https://medicalxpress.com/information/2023-12-dynamic-picture-high-oxygen.html
This doc is topic to copyright. Aside from any truthful dealing for the aim of personal research or analysis, no
half could also be reproduced with out the written permission. The content material is offered for info functions solely.