WASHINGTON — The federal authorities has burned by means of greater than $1 billion to check lengthy Covid, an effort to assist the hundreds of thousands of People who expertise mind fog, fatigue, and different signs after recovering from a coronavirus an infection.
There’s mainly nothing to point out for it.
The Nationwide Institutes of Well being hasn’t signed up a single affected person to check any potential remedies — regardless of a transparent mandate from Congress to check them. And the few trials it’s planning have already drawn a firestorm of criticism, particularly one intervention that specialists and advocates say may very well make some sufferers’ lengthy Covid signs worse.
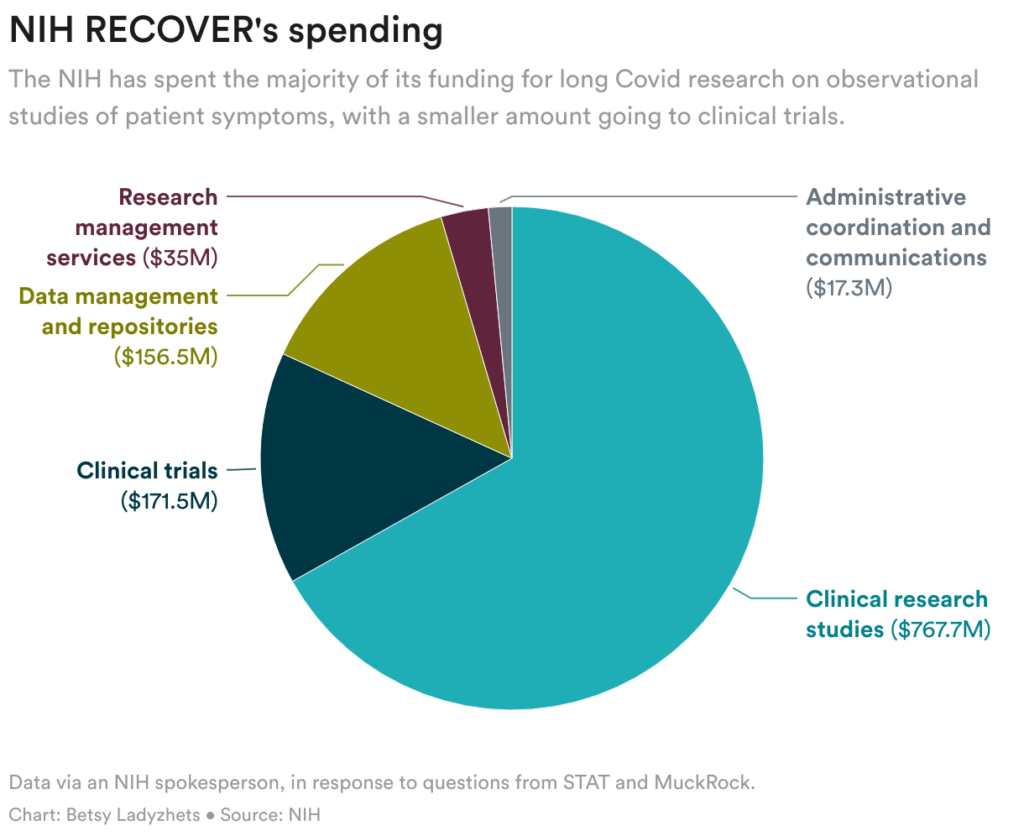
As a substitute, the NIH spent the vast majority of its cash on broader, observational analysis that received’t straight carry aid to sufferers. Nevertheless it nonetheless hasn’t printed any findings from the sufferers who joined that examine, nearly two years after it began.
There’s no sense of urgency to do extra or to hurry issues up, both. The company isn’t asking Congress for any extra funding for lengthy Covid analysis, and STAT and MuckRock obtained paperwork exhibiting the NIH refuses to make use of its personal cash to alter course.
“To this point, I don’t suppose we’ve gotten something for a billion {dollars},” stated Ezekiel Emanuel, a doctor, vice provost for international initiatives, and co-director of the Healthcare Transformation Institute on the College of Pennsylvania. “That’s simply unacceptable, and it’s a critical dysfunction.”
Eric Topol, the founder and director of the Scripps Analysis Translational Institute, stated he anticipated the NIH would have launched many large-scale trials by now, and that testing remedies ought to have been an pressing precedence when Congress first gave the company cash in late 2020.
“I don’t know that they’ve contributed something besides extra confusion,” Topol stated.
Sufferers and researchers have already raised alarms concerning the glacial tempo of the NIH’s early lengthy Covid efforts. However a brand new investigation from STAT and the nonprofit information group MuckRock, based mostly on interviews with practically two dozen authorities officers, specialists, sufferers, and advocates, and inside NIH correspondence, letters, and public paperwork, underscores that the NIH hasn’t picked up the tempo — as a substitute, the delays have compounded.
It’s tough to pinpoint precisely why progress is so stalled, specialists and sufferers concerned within the challenge emphasised, as a result of the NIH has obscured each who’s in control of the lengthy Covid efforts and the way it spent the cash. The broader Biden administration has additionally missed alternatives for oversight and accountability of the hassle — regardless of the president’s lofty guarantees to deal with the illness.
The NIH’s blunders have huge ramifications for the greater than 16 million People affected by lengthy Covid, along with these with different, related power illnesses. As the most important government-funded examine on this matter, the NIH initiative, dubbed RECOVER, units precedents for future analysis and medical pointers. It is going to dictate how medical doctors throughout the nation deal with their sufferers — and, in flip, impression individuals’s means to entry work lodging, incapacity advantages, and extra.
“The NIH RECOVER examine is pointless,” stated Jenn Cole, an extended Covid affected person based mostly in Brooklyn, N.Y., who needed to enroll within the examine however discovered the method inaccessible. The analysis is “a waste of time and sources,” she stated, and fails to make use of sufferers’ tax {dollars} for his or her profit.
In response to STAT and MuckRock’s questions, the NIH and an institute at Duke College managing the medical trials defended the initiative, with out offering a transparent rationalization for the delays.
The NIH stated it selected to fund a large-scale analysis program as a substitute of small-scale research to verify information and processes could possibly be shared throughout completely different teams of sufferers, including that medical trials will probably be launching quickly. In these trials, standardized examine designs will enable the company to check a number of remedies throughout a number of websites. If there are alerts a drug works, the company stated it could possibly pivot to dedicate extra sources there.
A Division of Well being and Human Providers spokesperson stated the company has made progress during the last yr in responding to lengthy Covid, and that there are analysis efforts underway along with the RECOVER program.
“The Administration stays dedicated to addressing the longer-term impacts of the worst public well being disaster in a century,” HHS stated.
In 2020, Congress made an funding of $1.2 billion to be taught extra concerning the mysterious ongoing signs that had been afflicting some individuals contaminated with Covid-19. That kind of cash to fund analysis right into a power situation like lengthy Covid was just about extraordinary.
The cash was explicitly earmarked to fund each analysis to grasp the illness and medical trials to check remedies that would carry sufferers aid. However greater than two years in, the company hasn’t began testing a single remedy. Neither is it planning to check many sooner or later. As a substitute, it’s centered on observational analysis — and that, too, has produced few insights.
The NIH is planning 5 medical trials, every of which can check remedies that will assist with a significant class of lengthy Covid signs. A few of these remedies will probably be medication, whereas others will probably be behavioral therapies, equivalent to cognitive retraining. Every trial will embody 300 to 900 sufferers, chosen based mostly on their signs, based on particulars shared throughout a webinar in mid-April.
The one trial to be formally introduced up to now will deal with Paxlovid, testing whether or not the drug alleviates signs by mitigating any ongoing viral an infection in sufferers’ our bodies. The examine was supposed to start out recruiting in January.
However as of April, RECOVER hasn’t signed up a single affected person for any of these medical trials. And the timeline has slipped time and again.
Initially, in a letter to members of Congress prompted by STAT’s March 2022 reporting on the initiative’s gradual begin, the NIH instructed lawmakers that the company anticipated to launch medical trials by that fall. However by August, the estimated launch had slipped to “by the top of 2022.” Then, one other delay grew to become public in December, when one of many NIH officers main RECOVER instructed advisers that medical trials would start by the primary quarter of 2023. Now, Duke College, which is overseeing the medical trial infrastructure, instructed STAT and MuckRock it expects the primary sufferers to enroll in trials this summer season.

Emanuel stated the tempo of trials reveals little urgency on the a part of NIH.
“When you don’t have the pathobiology found out, you attempt issues. You don’t simply gradual, gradual, gradual, stroll it,” he stated.
All 5 medical trial protocols are going by means of security opinions, and the Meals and Drug Administration is reviewing the trials that can check Paxlovid and different medication, the Duke Scientific Analysis Institute stated. The institute plans to share these protocols publicly when opinions are full, however didn’t present an estimate for when that can occur.
Sooner progress is feasible. The same examine at Stanford, which obtained funding straight from Pfizer, was additionally introduced in October 2022 however has already begun recruiting sufferers. This trial was “in a position to be extra versatile and get the examine began sooner” compared to RECOVER as a result of it’s smaller, stated Upinder Singh, the examine’s principal investigator. Singh and her colleagues are solely testing Paxlovid and doing so at just one location, reasonably than evaluating completely different remedies.
Duke was additionally presupposed to create a affected person registry to gather details about lengthy Covid sufferers, however that initiative hasn’t been launched, both.
“A affected person registry remains to be deliberate, however the scope is being reassessed to most successfully meet the wants of the Initiative,” Duke stated.
Quite than prioritizing remedies from the beginning, the NIH used a lot of its lengthy Covid funding on a large-scale examine to trace lengthy Covid signs and find out how the illness works. This selection has pissed off sufferers as a result of 1000’s of different research have already answered many main questions concerning the situation.
“We didn’t have to recreate” present research that already answered these questions, stated Cole, the lengthy Covid affected person. Researchers have been compiling lists of widespread signs since summer season 2020, she stated. For Cole, fatigue and mind fog are probably the most debilitating features of the situation.
And even the symptom examine is shifting slowly, partially as a result of the initiative has failed to herald wholesome individuals who could possibly be in contrast towards lengthy Covid sufferers. RECOVER shortly stuffed its slots for individuals who had Covid greater than 30 days previous to their recruitment, however remains to be searching for individuals who had been contaminated lately, examine lead Leora Horwitz stated in a press release. Most examine websites closed enrollment for lengthy Covid sufferers on the finish of August 2022.
Nearly all of the scientific findings to emerge from RECOVER up to now have been based mostly on small teams of sufferers or on digital well being information, reasonably than on the 1000’s of people that signed as much as take part.
The crawling tempo of the federal government’s lengthy Covid efforts stand in stark distinction with the federal government’s wildly profitable partnership with the pharmaceutical business to get Covid-19 vaccines to market in lower than 12 months. There are not any ongoing efforts to help unbiased private-sector corporations or researchers attempting to check remedies for lengthy Covid by means of the NIH, though some have proved promising. Simply this month, the White Home left lengthy Covid out of a $5 billion effort to analysis next-generation Covid-19 remedies and vaccines.
Lengthy Covid researchers really feel there must be better urgency. Singh in contrast the strain that she’s at present beneath to the strain many scientists confronted earlier within the pandemic when finding out vaccines and coverings. “We as a scientific group have to deal with lengthy Covid and discover options for lengthy Covid,” she stated.
Topol echoed this sentiment, citing a latest opinion piece in Scientific American that known as for an Operation Warp Velocity for lengthy Covid remedies. “That’s what ought to have occurred,” he stated.
It’s nearly unimaginable to inform the place the NIH’s $1.2 billion pot of lengthy Covid cash has gone.
There is no such thing as a single NIH official liable for main RECOVER, and the initiative has did not share primary data that might usually be obtainable for a authorities analysis challenge of this scale.
In contrast to Operation Warp Velocity and different Covid efforts, the NIH has outsourced a lot of the work of working RECOVER to exterior organizations. New York College, RTI Worldwide, Mayo Clinic, Massachusetts Normal Hospital, and Duke College are liable for varied elements of the initiative.
Most of the analysis tasks related to RECOVER have been funded by means of these organizations reasonably than straight from the NIH. This course of makes it arduous to trace how choices are made or how cash is spent by means of public databases, stated Michael Sieverts, a member of the lengthy Covid Affected person-Led Analysis Collaborative who has a background in federal budgeting for scientific analysis.
Public information requests that MuckRock filed to the company in late 2022, supposed to reply questions on RECOVER’s funding, are nonetheless incomplete as of mid-April. Sieverts has equally requested inquiries to NIH officers and obtained no responses.
The group of RECOVER itself is convoluted, and tough to determine even for affected person advocates who’re straight concerned, they stated. It’s suggested by a posh sequence of committees, a few of which aren’t even posted on the initiative’s web site. There’s nobody individual in the end liable for coordinating among the many completely different institutes — and requests for details about the management hierarchy have been ignored.
“They don’t have an org chart for all the factor that exists, after two-plus years,” stated Diana Güthe, the founding father of Survivor Corps and a RECOVER adviser who has requested at practically each assembly she’s attended.
Lauren Stiles, a affected person advocate and President and CEO of Dysautonomia Worldwide who serves on a number of RECOVER committees, shared related considerations.
“There’s an entire lack of transparency. After we ask who made this choice … they received’t inform us,” Stiles stated.
In consequence, when RECOVER says it’s working out of funds, it’s arduous to establish who’s liable for main choices.
In response to questions concerning the initiative’s finances, the NIH stated it has no cash obtainable for extra programming. The company stated $811 million has been legally dedicated to varied actions, and the remaining is earmarked to help future analysis actions.
The finances restrictions are having sensible impacts already.
A RECOVER advisory committee liable for rating and evaluating potential remedy choices was placed on hiatus “as a result of an absence of funds,” the committee’s chief instructed members in late January, per an e-mail trade shared with STAT and MuckRock that has not been beforehand reported.
The NIH instructed STAT and MuckRock that the committee was paused as a result of the medical trial medicines, units, and remedy applications have been chosen. Nonetheless, the company stated that the RECOVER medical trials are “adaptive platform trials,” which suggests they’re designed with the intention of eradicating and including remedies as new data turns into obtainable.
This present finances squeeze didn’t come with out warning: The NIH was well-aware final summer season that the company wouldn’t have the funds for to run medical trials that matched the initiative’s objectives of reaching sufferers with numerous signs.
One in every of RECOVER’s co-chairs wrote to Congress in June that “further sources are vital” to check the complete vary of remedies wanted.
However the Biden administration isn’t taking any motion to get extra funding throughout the company, or from lawmakers.
NIH appearing Director Lawrence Tabak instructed affected person advocates that the company isn’t planning on directing any additional funding for RECOVER throughout the company. The company stated that such a request would probably undercut a failed request for supplemental funding that Congress ignored final yr.

The Biden administration didn’t request any new funds for RECOVER in its 2024 finances, a largely aspirational doc that displays the administration’s monetary priorities.
The finances did embody $130 million in lengthy Covid-related asks for different businesses, together with for the Well being Assets and Providers Administration to help look after lengthy Covid sufferers with complicated wants and to coach main care suppliers, and for the Company for Healthcare Analysis and High quality to analysis the supply of lengthy Covid care and to ascertain lengthy Covid care hubs.
There’s additionally little accountability for NIH leaders to reveal how funds are spent or reply to different considerations with RECOVER as a result of an entity supposed to supervise lengthy Covid analysis throughout the federal authorities hasn’t been created.
In April 2022, President Biden issued a presidential memorandum calling on federal businesses to “harness the complete potential” of the federal government, in partnership with non-public sector companions, to answer lengthy Covid.
The follow-through has been missing on the initiative’s highest-profile objective.
In August, in a congressionally mandated nationwide lengthy Covid analysis plan, the Biden administration stated it might create an Workplace of Lengthy Covid Analysis and Apply at HHS. This month, HHS put out a reality sheet touting the administration’s progress in reaching its objectives — and omitted any point out of the workplace.
An HHS spokesperson stated that the division is working to develop the workplace, and requested funding in subsequent yr’s White Home’s finances for the Workplace of the Assistant Secretary for Well being to coordinate response efforts to lengthy Covid.
“It appears to have been like, nicely, if we don’t do something, possibly nobody will discover,” stated Güthe. “It’s so essential to do an analysis of what was promised. What’s been achieved, and what hasn’t?”
A enormous chunk of funding to check a power sickness like lengthy Covid is uncommon, so any medical trials that the NIH chooses to run are essential decisions — and a few medical doctors and advocacy teams have voiced critical considerations concerning the number of one medical trial specifically.
That trial would check train as a possible lengthy Covid remedy, regardless of years of analysis suggesting that train may hurt sufferers and set again additional examine.
Many individuals with lengthy Covid have related signs to individuals with myalgic encephalomyelitis or power fatigue syndrome (ME/CFS), a debilitating situation that usually follows viral an infection. The defining characteristic of ME/CFS is intense fatigue and worsening of different well being points after bodily or psychological exercise. This symptom, referred to as post-exertional malaise, usually happens with a lag, which may make it robust for medical doctors to diagnose — and even for sufferers to acknowledge themselves.

“What usually occurs is, individuals will go for a stroll, they could not really feel it for a day or two, after which all of a sudden, they really feel ailing on the third day,” stated Adam Lowe, an ME/CFS affected person and one of many founders of MEAction UK, the U.Okay. department of the Myalgic Encephalomyelitis Motion Community. Sufferers may all of a sudden change into bed-bound and have hassle focusing, he stated.
This worsening of signs occurs as a result of a affected person isn’t producing and utilizing vitality in the identical approach as a wholesome individual, stated Todd Davenport, a professor at College of the Pacific who has studied train and this situation. It’s an inside change much like the whole-body exhaustion {that a} marathon runner may expertise on the end line of their race.
A variety of previous research and surveys of sufferers have demonstrated how harmful train might be for individuals with ME/CFS. Many sufferers instructed to train by their medical doctors later dropped out of research or remedy regimens, citing worsening signs. One notorious trial that pointed to train as a possible remedy was later discredited as deeply flawed.
Learning train as a remedy may “body lengthy Covid as one thing that may be overcome with grit and arduous work,” stated Jaime Seltzer, the director of scientific and medical outreach at MEAction, arguing that such framing is “unsound and ethically troubling.”
Not all sufferers with lengthy Covid expertise post-exertional malaise, and people who don’t may discover train useful, Davenport stated. In these circumstances, gradual and cautious train by means of a rehabilitation or bodily remedy program may assist restore vitality techniques which have fallen off form.
However it might be tough to differentiate between these completely different teams of sufferers, except a medical trial is ready up with the utmost warning. “Ideally, what you’d need is a really coherent, very particular set of inclusion and exclusion standards,” Davenport stated. In any other case, the examine would danger producing outcomes that oversimplify lengthy Covid, he added, main medical doctors to broadly prescribe a remedy that doesn’t work for some or many.
Scientists and affected person advocates liable for advising RECOVER have warned that an train trial may hurt sufferers, however obtained combined responses. Sufferers concerned within the examine despatched emails and social media posts demanding that RECOVER cease the deliberate trial, whereas MEAction despatched a public letter to NIH leaders.

Scientists and clinicians on an NIH advisory committee centered on rehabilitation equally advised that post-exertional malaise could possibly be a harmful results of the trial, based on inside emails shared with STAT and MuckRock. In response, NIH program officer Antonello Punturieri pushed again on the considerations. Punturieri cited medical pointers from the World Well being Group and a U.Okay. company, though each advocate towards train for individuals with ME/CFS.
In response to those considerations, RECOVER arrange inside conferences together with researchers in control of the train examine, affected person representatives, and the initiative’s high advisory committee. “Work is now underway to additional revise that protocol” based mostly on these conferences, the Duke Scientific Analysis Institute stated.

The examine’s deliberate revisions will handle considerations about affected person security, equivalent to monitoring for post-exertional malaise after train. Nevertheless it’s unclear how the researchers will do that screening, or whether or not ME/CFS medical doctors will probably be concerned.
Even with revision, specialists and affected person advocates stay involved that the train examine takes sources away from different analysis and will result in dangerous suggestions from medical doctors. If RECOVER finds train is useful for some sufferers, requested JD Davids, an advocate with Lengthy COVID Justice and creator of a petition asking the NIH to cease this trial, “What are the possibilities that medical doctors would accurately perceive how restricted this advice is? I feel it’s very low.”
It’s not like there aren’t loads of potential remedy choices price finding out.
Topol and different researchers compiled a full desk of different remedy candidates for a evaluate paper printed in Nature in January. Specialists on one in all RECOVER’s advisory committees compiled an identical checklist, for a paper printed in March.
Given “the variety of different candidate remedies on the market, I can’t think about why you’d select graded train remedy,” stated Julia Moore Vogel, a researcher on the Scripps Translational Institute dwelling with lengthy Covid, and co-author of the Nature evaluate paper. Vogel is main a examine of wearable units for lengthy Covid, which can begin with about 500 contributors regardless of planning for as much as 100,000.
One examine has even reported outcomes already, through a preprint shared by The Lancet in early March. The trial discovered that metformin, a standard remedy for diabetes that additionally has antiviral properties, lowered Covid sufferers’ danger of creating long-term signs by about 42%.
This analysis group truly didn’t got down to examine lengthy Covid, stated David Boulware, one of many scientists and an infectious illness doctor on the College of Minnesota Medical College. The preliminary objective was to guage potential remedies for acute Covid-19, however the workforce added lengthy Covid monitoring partway by means of the trial.
And it’s unlikely to get additional examine with out some form of authorities help. The preliminary examine relied on philanthropic funding, and extra grants could be wanted to maintain finding out this generic drug.
“It’s an excellent drug, it’s low-cost, it’s obtainable worldwide,” Boulware stated, “however there’s no revenue margin for anybody to check it.”
There could also be related considerations for analysis into low-dose naltrexone, an off-label use of the dependancy drug that has change into widespread for lengthy Covid and different power illnesses. In low doses, naltrexone might help scale back irritation within the immune and neurological techniques, probably assuaging lengthy Covid signs.
However as a result of the drug has been broadly obtainable for many years, pharmaceutical corporations aren’t motivated to fund giant trials. Just a few small medical trials are underway, based on reporting by Rolling Stone.
The shortage of assist from NIH has left biotech executives pissed off.
“You must perceive what you’re attempting to sort out, so we help that, after all. However as sufferers will inform you, we would like intervention, not remark,” stated Axcella CEO Invoice Hinshaw. His Massachusetts-based firm has gone all in on testing a drug candidate to deal with lengthy Covid signs, with none assist from NIH.
Tonix Prescribed drugs, which is creating a fibromyalgia remedy that the corporate is hoping could possibly be an efficient remedy for lengthy Covid signs, didn’t obtain any funding from NIH both, regardless of placing in an software.
“I hope there are extra therapeutics trials. And I feel that the therapeutics trials can go hand in hand with the pure historical past form of research like RECOVER,” Tonix CEO Seth Lederman stated.
Sufferers and specialists worry that if RECOVER is the extent of federal effort to check lengthy Covid, the situation may fall into the longstanding sample of apathy and lack of urgency that has made breakthroughs in power sickness remedy difficult.
“It’s clear that there are lots of people on the NIH who’re devoted and decided, attempting to determine this out,” stated Charlie McCone, a affected person consultant at RECOVER. In consequence, “sufferers are confused” why solely a handful of medical trials have been deliberate and none of these have launched but, he stated.
Because the NIH initiative drags its toes, sufferers are left largely on their very own to analysis potential remedies, stated Cole, the Brooklyn-based affected person, who has been scuffling with signs since April 2020. “As a result of we’re not funding these promising remedies, and we’re not disseminating them by means of the medical system, it’s left to me to determine methods to make that occur for myself,” she stated.
Cole, like many others within the lengthy Covid group, feels deserted by the federal authorities and well being care system at giant. If her signs worsen to the purpose that she will be able to now not work, she stated, “the system’s not going to be there to choose me up.”
This text was co-reported with MuckRock, a nonprofit newsroom that brings collectively journalists, researchers, activists, and common residents to request, analyze, and share authorities paperwork. Join its newsletters right here.
Funding for this challenge additionally got here from Columbia College’s Brown Institute for Media Innovation.